Select a Statement That Best Describes the Oxidation Process
O Oxidation results in a loss of electrons so the oxidation number decreases O Oxidation results in a gain of. C the last intermediate is a 3 carbon propionlycoA.

Coordination Compounds Ligands And Complex Ions Chemistry Notes Transition Metal Teaching Chemistry
Sana po may sumagot po.
. A change in _____ means that a chemical reaction has occurred. E gain of elections. Home Store All Categories Books.
Which statement describes the process of oxidation. Sana Po may sumagot po. The type of reaction that is shown is.
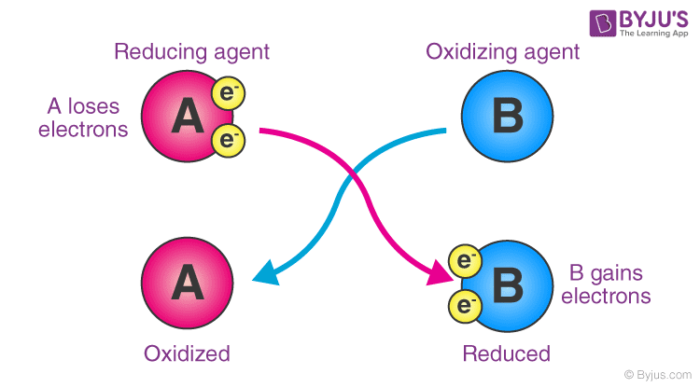
However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. Chemistry 02122021 2240 kamillecronk. The chemical formula that shows the correct subscripts is D BeF₂.
Oxidation results in a gain of electrons so the oxidation number decreases. E in oxidation process some eleents. 1 point Oxidation results in a loss of electrons so the oxidation number increases.
A chemical reaction in which electrons are transferred between reactants. Oxidation results in a loss of electrons so the oxidation number decreases. Which statement BEST describes the process of oxidation.
Which statement best describes the oxidation of odd numbered fatty acids. Decrease in oxidation number. Which statement describes the process of oxidation.
C decrease in oxidation number. B in oxidation process all elements experience an increase in oxidation state. Does 2 explains the reason for the 1st one or is it because because of Fs higher electronegativity.
Oxidation results in a gain of electrons so the oxidation. Start studying the Chapter 11 Quiz flashcards containing study terms like Select the statement that best describes the antimicrobial activity of chlorhexidine Choose the method used to sterilize an inoculation loop used in lab for culturing bacteria The easiest microbial forms to kill or inhibit. Oxidation results in a gain.
1- All except one are true about gastriclipase-a Main preduodenal lipaseb Hydrolyzes triglycerides containing shortand medium chain fatty acidsc Primary site of hydrolysis is sn-3ester linkaged Optimum pH is 60-702- Pancreatic juice does not contain which ofthe followings-a Bile saltsb Phospholipase A2c Lipase and colipased Lipoprotein. Oxidation is the rapid decomposition of organic materials. O Oxidation results in a loss of electrons so the oxidation number increases.
But when an element is reduced it gains electrons. D loss of electrons. 2 which statement best describes a social contract Aan implied agreement between citizens and government in which citizens release some liberty to government for the good of society Ba contract between local residents and providers of basic services.
Which statement describes the process of oxidation. See answer 1 Best Answer. A multistep process resulting in the enzymatic splitting and oxidation of glucose to form pyruvic acid with a net gain of 2 ATP The chemicals in which energy is stored in cells isare ________.
A addition of hydrogen. Choose the statement that best describes glycolysis. Oxidation is the process of losing one or more electrons.
Select a statement that best describes the oxidation process A In oxidation from CHEMISTRY 1142 at Troy University. Memorize flashcards and build a practice test to quiz yourself before your exam. Oxidation is when a substance catches fire spontaneously.
Oxidation is a process of gaining one or more electrons. Oxidation is the process of losing one or more electrons. Oxidation results in a gain of electrons so the oxidation number decreases.
O Oxidation results in a loss of electrons so the oxidation number decreases O Oxidation results in a gain of electrons so the oxidation number decreases O Oxidation results in a gain of electrons so. Which statement describes the process of oxidation. A Redox reaction oxidation-reduction reaction involves the exchangetransfer.
O Oxidation results in a loss of electrons so the oxidation number increases. Which statement best describes the differences between architecual developments in Russian and Inca culture. D in oxidation process all elements change oxidation state.
1In the reaction O22F2-- 2 OF2 F2 is the oxidizer. When an element is oxidized it loses electrons. Oxidation results in a loss of electrons so the oxidation number decreases.
D one carbon is removed in the first cycle. B the human body cannot naturally metabolise odd numbered fatty acids. Oxidation is a process of gaining one or more electrons.
Which statement BEST describes the process of oxidation. Meanwhile a reducing agent reduces something else and gets oxidized in the process losing its own electrons. Which statement BEST describes the process of oxidation.
Oxidation is the rapid decomposition of organic materials. Oxidation is when a substance catches fire spontaneously. Which statement BEST describes the process of oxidation.
C decomposition reaction. Which statement BEST describes the process of oxidation. C0 in oxidation process only oxygen increases its oxidation state.
See below for the correct answer. Select a statement that best describes the oxidation process. 2F cant have positive oxidation states.
1 point Oxidation results in a loss of electrons so the oxidation number increases. The correct statement that describes a Redox reaction is D. Combustion is basically OXIDATION.
A the last intermediate cannot be used by humans. Remember me Sign in. 100 correct and accurate.
Which statement BEST describes the process of oxidation. A in oxidation process some elements experience oxidation state increase. This is the basis of redox reactions.
B loss of oxygen.

Manufacturing Tea Ielts Writing Writing Tasks Ielts

Oxidizing Agent Definition Properties Examples Applications
Solved Which Of The Following Statements Correctly Describe Chegg Com
Comments
Post a Comment